The Joint Access Advisory Mechanism (JAAM)
The Joint Access Advisory Mechanism (JAAM)
- The JAAM activities were discontinued in December 2024. -
The mission of the JAAM is to ensure, through an independent panel of experts, a solid scientific assessment of the most promising candidate treatments that meet the needs of the various patient populations and of the health systems, and are adapted to the design and capacities of the EU-funded adaptive platform trials in emerging infectious diseases.
Led by: ECRIN
The JAAM is constantly evolving to meet the current needs of pandemic preparedness with an initial focus on COVID19, the remit of the JAAM is in the process of being expanded to all current and future EU-funded pandemic preparedness platform trials.
The JAAM is composed of an expert panel responsible for the independent scientific evaluation of treatment / vaccine candidates submitted to the JAAM secretariat
How does it work?
The JAAM’s role is limited to the provision of access through independent scientific assessment and recommendations for prioritization.
The JAAM meets regularly, to assess requests for access according to the following criteria :
- Public health interest;
- Scientific, medical and ethical soundness;
- Appropriate patient population, comparator and outcomes;
- Promotion of coordination and optimal use of resources.
Following its assessment, the JAAM emits recommendations to initiate negotiations with the trials, and the final decision on access is made by the Trial Steering Committee of each individual trial.
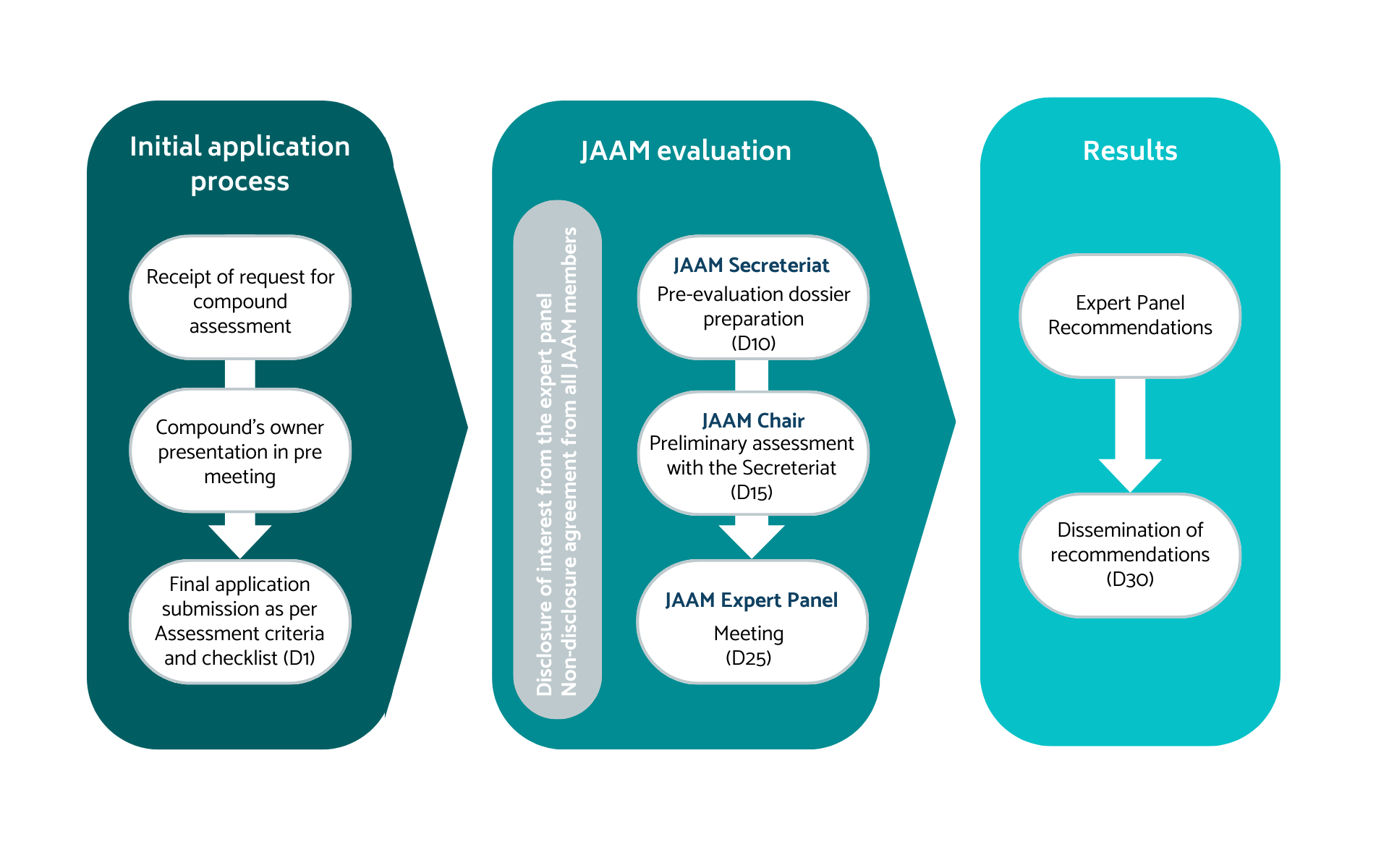
Process and Timelines
Any independent investigator or company (pharmaceutical, SMEs etc) can request access to the EU APTs via the JAAM, to assess their repurposed or new investigational drug.
The applicant must submit their proposal to the JAAM Secretariat at jaam@platformtrials.com. Applications are intended to be prepared for a phase II trial with supporting documents from the earlier work.
The secretariat is available to discuss the best timing for submission. Early contact with the secretariat is recommended if you have a clear plan for drug development and you are willing to receive follow-up emails.
Applicants will be requested to cover all key characteristics of the compound and use the JAAM checklist to ensure all necessary elements are included in their submission. The JAAM Secretariat will then ensure all information provided by the applicant is complete (and may request additional information), and will consult the JAAM chair for eligibility of the compound for expert panel JAAM evaluation.
After which, the Secretariat will organize a meeting of the JAAM, where the applicant will be able to present their proposal to the JAAM expert panel. Following the applicant's presentation, the JAAM will deliberate and emit a recommendation for the applicant to begin negotiations with one or more of the adaptive platform trials.
The JAAM Secretariat will inform the applicant of the recommendation within five working days of the JAAM meeting. The full cycle between the complete application submission and the notification of the applicant by the JAAM is around 30 working days.
Other JAAM Activities
The JAAM now conducts proactive horizon scanning, identifying compounds of potential interest in the development pipeline, then reaching out to the industry or academic developers to explore their interest for accessing an adaptive platform trial.
This horizon scanning will be conducted by the JAAM secretariat through scientific literature and grey literature review, requests to clinical trial registries, metadata repositories (https://newmdr.ecrin.org), and other horizon scanning tools (www.ihsi-health.org), with the support of HERA, EMA, the Coordinating Committee, and of members of the JAAM expert panel.
Evolution
The JAAM activities were discontinued in December 2024.


